Apr 09, 2024, 4:00 am UTC
4 min
Created by
A guide to the different types of Alzheimer’s disease
From person to person, Alzheimer’s looks similar, but there are four main types of the disease. As scientists unravel the disease’s biology, that number may rise.
Alzheimer's disease (AD) was once perceived as a monolith. However, this progressive neurodegenerative disorder comes in various forms that kick off when abnormal protein deposits — beta-amyloid plaques and tau tangles — begin to buildup in the brain, killing off nerve cells and severing neural connections. In time, this neurodegeneration leads to dementia.
As the disease progresses, patients experience a range of symptoms from memory loss, confusion, and difficulty with communication to changes in mood and behavior. While the effects of look similar, a new study by a team of Dutch researchers has found five different biological subtypes of the disease — each with a distinct molecular makeup and risk profile.
This new way of characterizing the disease based on different biological mechanisms that may be driving it — hyperplasticity, innate immune activation, RNA dysregulation, choroid plexus dysfunction, and blood-brain barrier dysfunction — is one of many efforts to redefine the disease and move beyond the categories researchers and clinicians have relied upon for decades.
Potential molecular mechanisms of Alzheimer’s
A recent study suggests there may be five molecular mechanisms driving Alzheimer's disease. If the results can be replicated, they have implications for how the most common form of dementia is diagnosed and treated.
Hyperplasticity – characterized by higher levels of brain cell growth and tau protein levels.
Innate immune activation – characterized by an overactive innate immune system leading to greater brain atrophy and elevated tau levels.
RNA dysregulation – characterized by the dyregulation of RNA, which impairs the brain’s production of proteins.
Choroid plexus dysfunction – characterized by the disruption of the brain’s blood vessels and extracellular matrix and slower brain cell growth, leading to brain atrophy.
Blood-brain barrier dysfunction – characterized by a breakdown of the blood-brain-barrier, a protective membrane that lines the brain’s blood vessels, leading to microbleeds and slower brain cell growth.
As scientists get closer to uncovering the biological basis of the disease, the subtypes still used to describe the condition today are likely to shift. Until then, here are the major faces of AD:
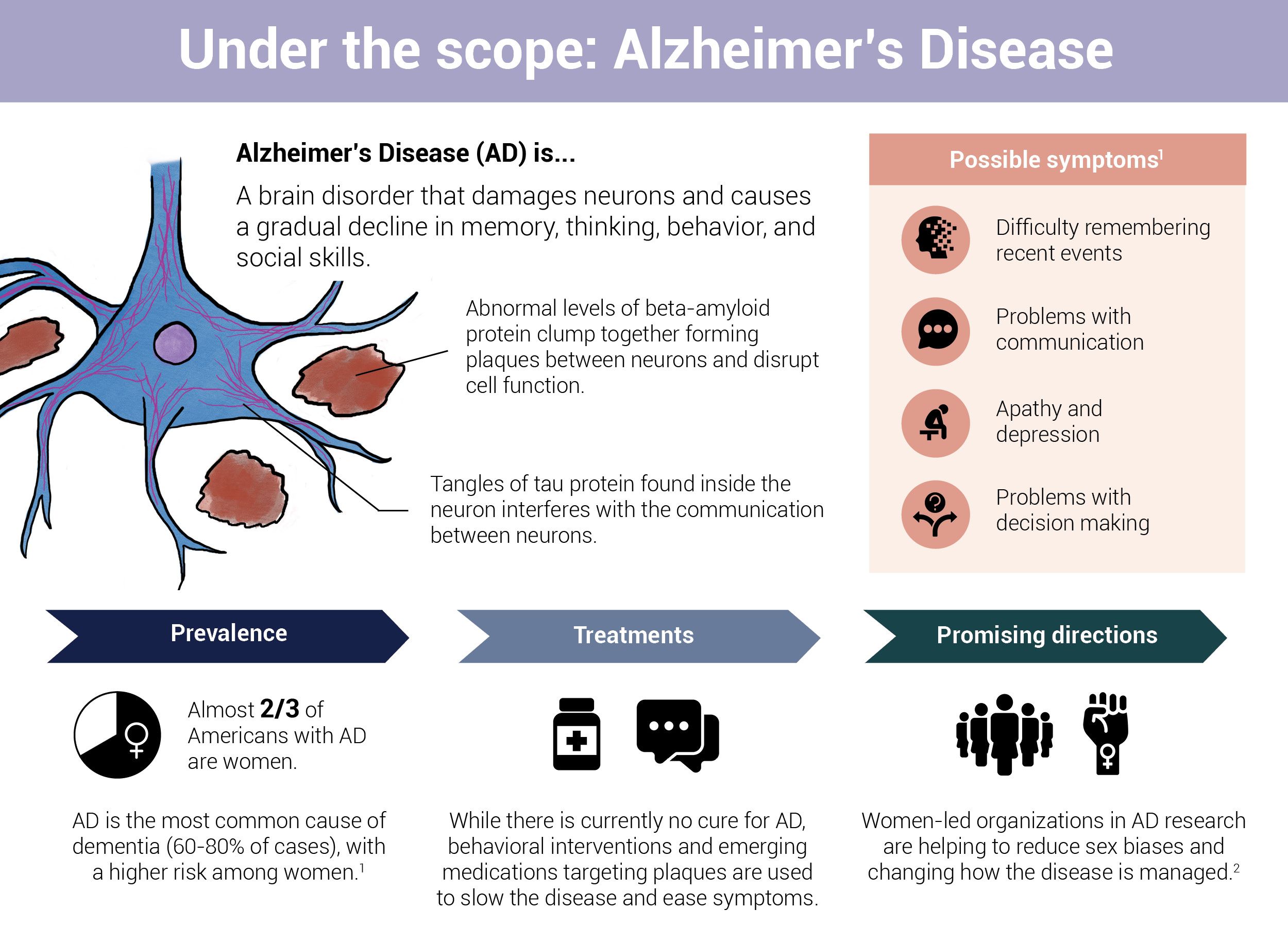
Familial Alzheimer's Disease (FAD)
This rare form accounts for less than 5% of all AD cases and is characterized by its early onset and strong genetic component. Unlike other types of Alzheimer’s that manifest around the age of 65, people with FAD begin to experience symptoms as early as in their 30s. Research suggests when a mutation in one of the three genes — amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) — that regulate the production of amyloid beta protein is inherited, it is highly likely the individual will develop FAD. This type of AD tends to have a rapid progression, with women experiencing more severe cognitive decline than men.
Distinct signs and symptoms: People with Familial Alzheimer's Disease (FAD) may experience unusual symptoms like seizures, and muscle stiffness and twitching, which are not typical in other types of Alzheimer's.
Diagnosis: FAD is typically diagnosed through genetic testing to identify mutations in genes like APP, PSEN1, and PSEN2. Clinicians also conduct cognitive assessments and brain imaging to confirm symptoms and structural brain changes characteristic of the disease. Genetic counseling is recommended for individuals undergoing genetic testing for AD to understand — and cope — with results.
 Infographic by Cat Lau. (References below.)
Infographic by Cat Lau. (References below.)Sporadic Alzheimer's Disease
This is the most common form of AD, accounting for the majority of Alzheimer’s cases globally. Symptoms of sporadic Alzheimer’s typically become noticeable around the age of 65, and the disease progression is generally slow but relentless. While both men and women are affected by this subtype, the condition is significantly more prevalent in women. The exact causes of sporadic AD are unclear, but studies suggest it’s a combination of genetic, environmental, and lifestyle factors.
Distinct signs and symptoms: Many individuals with sporadic AD initially experience mild cognitive impairment, where memory loss and other cognitive difficulties are noticeable but not severe enough to interfere significantly with daily functioning.
Diagnosis: Sporadic AD is diagnosed through a careful review of medical history, memory and thinking tests, and brain scans like an MRI. While genetic testing may also be conducted, it isn’t common as sporadic AD isn't caused by specific genetic mutation. Instead, diagnosis relies on identifying typical AD symptoms like memory problems and changes in thinking.
Suspected cases of AD are then corroborated with PET scans, a cerebrospinal fluid (CSF) analysis, or MRI scan. A PET scan is an imaging test that searches for proteins such as amyloid-beta and tau in the brain. CSF analysis involves examining a sample of fluid that surrounds the brain and spinal cord, known as cerebrospinal fluid. This fluid analysis can indicate the presence of elevated levels of proteins associated with Alzheimer's disease. An MRI can be used to looks for signs of neurodegeneration or neuronal injury and dysfunction.
The ATN System
In 2018, researchers from the U.S. National Institute of Aging Alzheimer's Association Research Framework released a biological definition of Alzheimer disease for research purposes. It marked a shift from defining the disease based on stages (preclinical, mild cognitive impairment (MCI), and dementia), to biological changes in the brain that can be detected using fluid and imaging biomarkers.
“A” stands for beta amyloid measured by PET scan or cerebrospinal fluid (CSF).
“T” stands for tau pathology measured by CSF or PET.
“N” stands for neurodegeneration or neuronal injury and dysfunction measured by MRI or CSF.
Early-Onset Alzheimer's Disease (EOAD)
This is another rare subtype of AD that affects individuals younger than 65 years old, with symptoms typically appearing between the ages of 30 and 60. While EOAD and Familial Alzheimer's Disease (FAD) may look similar, their progression differs — FAD advances more rapidly, causing faster cognitive decline and functional impairment.
EOAD, much like FAD, is also linked to mutations in genes controlling amyloid beta protein production. However, their inheritance patterns vary — EOAD can occur sporadically without a clear family history, while FAD follows an autosomal dominant pattern. This means that if an individual has a family history of FAD, there's a 50% chance they'll inherit the mutation and develop the disease.
Diagnosis: When diagnosing EOAD, clinicians consider a broader range of environmental and lifestyle factors alongside genetic testing, although genetic factors are typically less emphasized.
Late-Onset Alzheimer's Disease (LOAD)
Late-Onset Alzheimer's Disease (LOAD) is the most prevalent form of Alzheimer's, commonly affecting individuals aged 65 and older. Symptoms of LOAD progress gradually, with patients experiencing a slow decline in cognitive abilities over the course of several years.
While both LOAD and sporadic AD have genetic components, LOAD is notably influenced by the presence of the APOE ε4 allele, which plays a role in the metabolism of fats and cholesterol in the body. In contrast, sporadic AD is linked to a complex interplay of genetic, environmental, and lifestyle factors, with less emphasis on specific genetic mutations.
Diagnosis: LOAD is diagnosed similarly to sporadic AD, but clinicians typically place more emphasis on age-related risk factors and the APOE ε4 allele.
The path to Alzheimer’s treatments
Understanding the many faces of Alzheimer’s disease is essential for patients and their families to plan for what lies ahead, but it's also key for driving innovation in clinical research. By grouping patients based on their specific subtype during clinical trials, researchers can test treatments more precisely. This approach could bring us closer to developing drugs that can actually change how the disease progresses and, eventually, tailoring treatments to each individual. It’s trying to solve a puzzle – the more we understand about each piece, the closer we get to finding a solution.
Infographic References
- 2023 Alzheimer's Facts and Figures: Special Report, Alzheimer's Association (Accessed June 20, 2023).
- Castro-Aldrete L, Moser MV, Putignano G, Ferretti MT, Schumacher Dimech A and Santuccione Chadha A (2023) Sex and gender considerations in Alzheimer’s disease: The Women’s Brain Project contribution. Front. Aging Neurosci. 15:1105620.