Apr 19, 2024, 4:00 am UTC
5 min
Created by
Breakdown of the mitochondrial "cargo system" drives neurodegeneration
Beyond their role as energy factories, mitochondria shuffle fuel to distant locations within cells. A breakdown of this railway system may be involved in neurodegenerative conditions like Alzheimer's and Parkinson's.
At any given moment, every cell within the human body is diligently executing its specialized task. But just as cogs in a machine rely on a steady energy supply to fuel their movements, cells can only carry out their diverse functions if supplied with a constant influx of fuel. Miniature locomotives within cells, also known as the mitochondria, make this energy delivery possible.
These specialized compartments, or organelles, power every cellular activity from signaling to programmed cell death. But now, researchers are increasingly linking the malfunction of these locomotives to neurogenerative diseases like Alzheimer's disease and Parkinson's. This means that understanding mitochondrial dysfunction — and finding ways to mitigate it — holds promise for developing innovative therapies that can ultimately slow down the progression of neurodegenerative diseases.
A journey inside the mitochondria
Imagine mitochondria as compact, bean-shaped locomotives within a vast railway network. Each mitochondrion has an outer protective membrane and an inner membrane with intricate folds called cristae. Cristae divides the mitochondrion into smaller compartments, allowing it to churn out energy with remarkable efficiency. These cellular locomotives are also highly dynamic, constantly adjusting their structure and function through fission (division) and fusion (merging) to accommodate varying cargo loads that meet cells' energy demands.
Amidst this dynamic landscape, mitochondria produce adenosine triphosphate (ATP), the cell's primary energy currency, through a process called oxidative phosphorylation. It unfolds within the inner membrane of the mitochondria, where specialized chains of proteins break down glucose and fatty acids, releasing energy that is then used to synthesize ATP. This energy is then shuffled off to different parts of the cell to power activities like muscle contraction, cell communication, programmed cell death, and the production of essential molecules, such as proteins, DNA, and RNA.
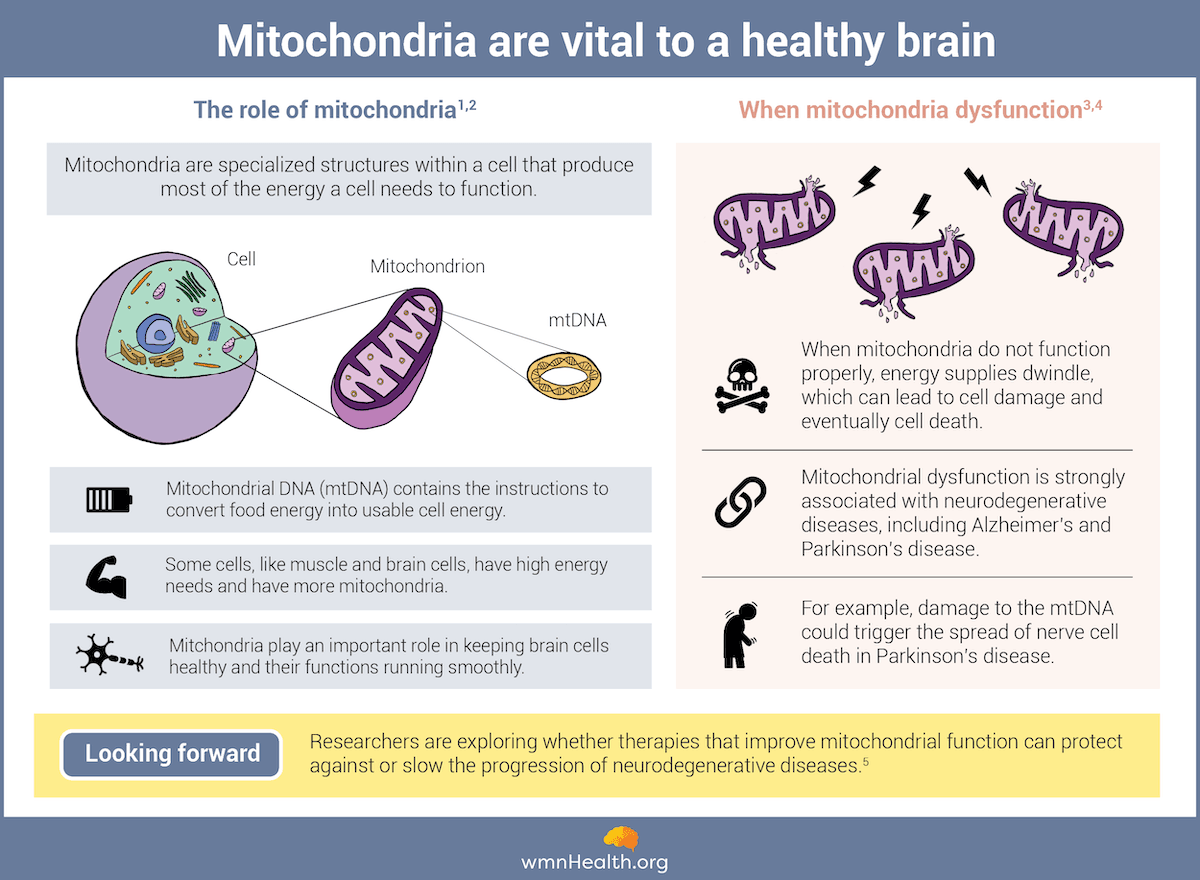 Infographic by Cat Lau. (References below.)
Infographic by Cat Lau. (References below.)What causes mitochondria to go awry — and what does that mean for brain function?
In brain cells, where energy demand is exceptionally high, mitochondria are vital for supporting neuronal function, enabling communication between brain cells, and producing chemicals essential for this communication.
When these organelles malfunction, neurons are left with reduced fuel to complete energy-intensive tasks like maintaining their health and transporting important substances along their axons. This energy deficit in the brain eventually causes problems with memory, learning, and overall cognitive function, signs and symptoms that arise as neurodegenerative diseases develop and progress.
Mitochondrial dysfunction can be caused by a variety of factors:
Genetic mutations: When genetic changes occur at the DNA in the cell's nucleus (nDNA) or in the DNA of the mitochondria themselves (mtDNA), they lead to defects in proteins critical for producing energy and transporting it to brain cells. These abnormalities disrupt communication between neurons by impairing synaptic transmission, the chemical event that allows neurons to send electrical signals to one another.
Environmental factors: Exposure to environmental toxins and pollutants can harm the mitochondrial outer membrane and ramp up the production of harmful molecules called reactive oxygen species (ROS) in the brain. These molecules are notorious for damaging brain cells by disrupting their ability to maintain a balanced internal environment and promoting inflammation and neuronal death.
Age-related changes: It's typical for mitochondria to accumulate damage over time. This decline in function means the organelles struggle to produce sufficient ATP, essential for keeping neurons alive and functioning. Age-related mitochondrial dysfunction can accelerate cognitive decline and render the brain more vulnerable to neurodegenerative diseases.
Mitochondrial dynamics dysregulation: This type of dysfunction occurs when processes such as mitochondria fission (division of one mitochondrion into smaller ones) and fusion (merging of multiple mitochondria into one) malfunction. This can lead to mitochondria becoming too small and weak to produce enough energy or forming larger mitochondria with disjointed halves that cannot function together.
Connecting mitochondrial dysfunction to neurodegenerative diseases
Eugenia Trushina, a professor of neurology at the Mayo Clinic in Rochester, Minnesota, has dedicated decades to studying mitochondrial dysfunction and its connections to neurodegenerative diseases. Her focus has finally begun to yield insights, with studies around the globe underscoring mitochondria's role in the onset and progression of conditions like Alzheimer's and Parkinson's disease.
In Alzheimer's disease, the buildup of proteins—specifically amyloid-beta and tau—in the brain is a key characteristic of the condition. Studies have found that these amyloid-beta aggregates can disrupt mitochondrial function in neurons by causing them to break apart and impair their ability to produce sufficient ATP. On the other hand, an excess of tau protein impacts mitochondria's ability to transport energy within neurons. It also induces excessive fission and fusion of mitochondria, which exacerbates oxidative stress and ultimately leads to cell death.
Similarly, in Parkinson's disease, the aggregation of alpha-synuclein protein and subsequent loss of dopamine-producing neurons in the brain's substantia nigra region are hallmark characteristics. Research indicates that these aggregated alpha-synuclein proteins directly interfere with mitochondrial function, disrupting energy production. Moreover, dysfunction in the mitochondrial quality control system contributes to the accumulation of damaged mitochondria in neurons, leading to their removal by the body.
What sex differences in mitochondrial function tell us about neurodegeneration
Fascinatingly, mitochondria have their own DNA, distinct from the DNA bound up in the nucleus of our cells. This DNA, also known as mitochondrial DNA or mtDNA, is inherited exclusively from the mother: During fertilization, the egg cell retains its mitochondria, while those from the sperm are left behind in the sperm tail. According to Trushina, this maternal inheritance pattern may be responsible for the notable differences in mitochondrial function between males and females.
"Mitochondria are adapted in the organism using the female hormonal landscape," she says. "Then, when a mother gives birth to a boy, these female mitochondria have to adapt to the male hormonal landscape, and that makes mitochondria work a little bit differently in males and females."
Indeed, studies have found variations in how mitochondria generate energy and activate cell death between males and females. These differences have big implications, particularly in brain injury or neurodegeneration, when the brain initiates a series of metabolic changes to improve cellular function and repair damaged tissues. While mitochondria mainly utilize fats to produce energy in females, these organelles rely on proteins in males. These fundamental differences in how cells obtain energy influence how well they survive following an injury.
"What it implies is that if energetic stress is the early event in any disease, like Alzheimer's, for example, males and females could respond very differently to [it]," Trushina adds. "Some mitochondria may be more adaptive and could be more robustly compensating for the disease."
Trushina's work has revealed that the female sex hormone, estrogen, may be underlying these sex differences in mitochondria function. While studying female mice undergoing menopause, the researchers found that glucose-dependent mitochondria start "cannibalizing" myelin lipids once estrogen levels decline. These lipids form a protective sheath around neurons, and their breakdown can lead to the degeneration of white matter in the brain. This process is a hallmark of conditions like Alzheimer's and multiple sclerosis, so the transition to menopause — and the subsequent changes in mitochondria metabolism — may be a factor increasing women's likelihood of developing neurodegenerative diseases.
"Two decades ago, mitochondria were considered a nuisance, and I was among the very few hooked on them. Now, these organelles are turning out to be more important than we've ever realized — a gift that keeps giving."
In a world where there are stark differences in how — and how many — men and women experience neurodegeneration, peering inside mitochondria could be the answer to transforming female brain health.
"Two decades ago, mitochondria were considered a nuisance, and I was among the very few hooked on them," says Trushina. "Now, these organelles are turning out to be more important than we've ever realized — a gift that keeps giving."
Infographic References
- Mitochondria. The National Human Genome Research Institute. (Accessed February 22, 2024)
- Functions of mitochondrial DNA. Biologywise. (Accessed February 22, 2024)
- Lindsey Shapiro, Mitochondrial DNA damage linked to spread of neurodegeneration. Parkinson's News Today. (Accessed February 22, 2024)
- Millichap LE, Damiani E, Tiano L, Hargreaves IP. Targetable Pathways for Alleviating Mitochondrial Dysfunction in Neurodegeneration of Metabolic and Non-Metabolic Diseases. Int J Mol Sci 22(21):11444 (2021).
- Bustamante-Barrientos FA, Luque-Campos N, Araya, MJ et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J Transl Med 21, 613 (2023).
Banner Image
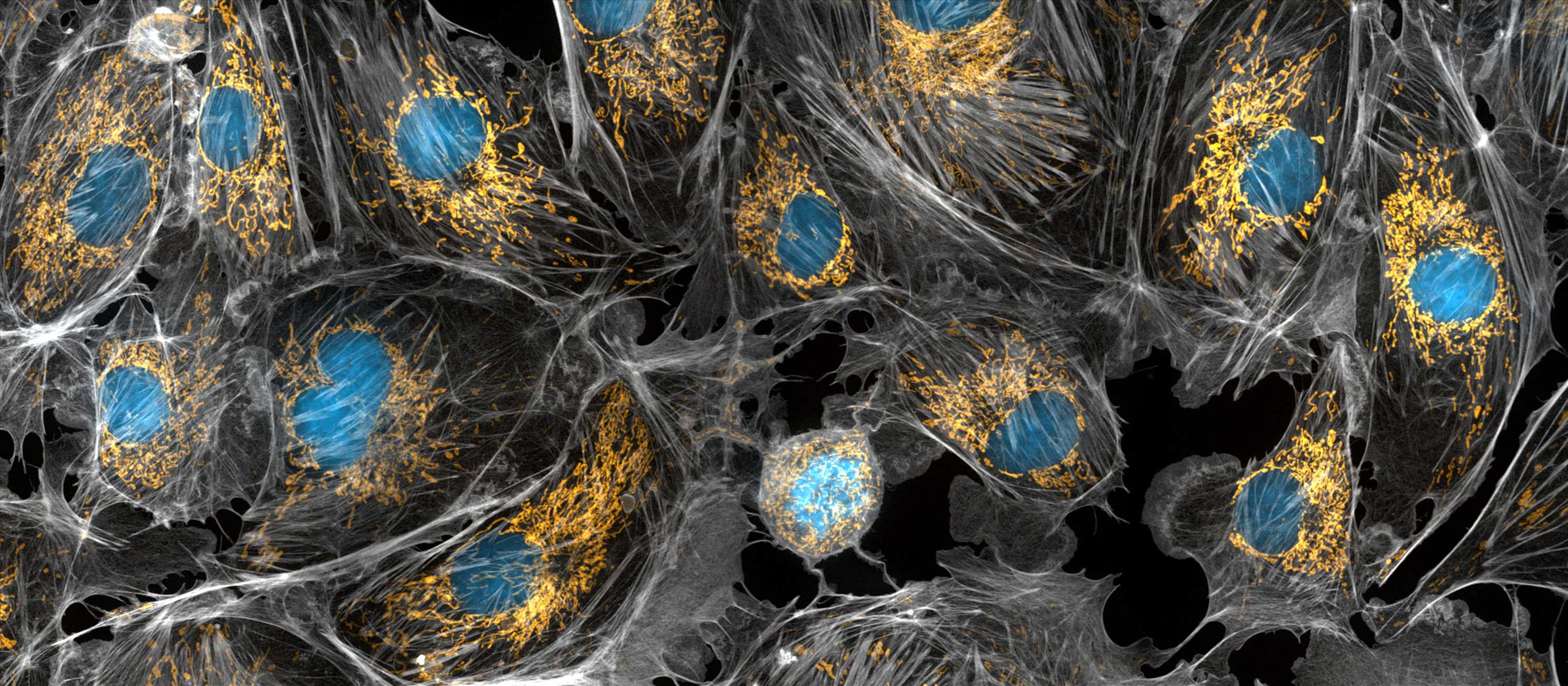
Mitochondria are the powerhouses of the cells, generating the energy the cells need to do their tasks and to stay alive. Researchers have studied mitochondria for some time because when these cell organelles don't work as well as they should, several diseases develop. In this photograph of cow cells taken with a microscope, the mitochondria were stained in bright yellow to visualize them in the cell. The large blue dots are the cell nuclei and the gray web is the cytoskeleton of the cells.
Credit: Torsten Wittmann, University of California, San Francisco