Feb 07, 2024, 5:00 am UTC
7 min
Created by
From clues to cures: Why biomarkers are essential to progress on neurodegenerative diseases
One of the top stories in neurodegenerative disease research last year was the discovery of a biomarker for Parkinson's disease. It was announced with fanfare by Michael J. Fox's Parkinson's research foundation, which has been supporting biomarker discovery research in Parkinson's for about two decades.
Biomarkers are potent indicators of a disease that hold the key to early detection, accurate diagnosis, and potential breakthroughs in treatment.
For decades, developing effective treatments for neurodegenerative diseases like Parkinson's, Alzheimer's, and Huntington's—affecting millions worldwide and still incurable—has been elusive, partly due to the complexity of the biological processes driving these diseases.
Now, the search for biomarkers for neurodegenerative diseases is finally paying off and catalyzing urgently needed progress in the field.
This article will break down the concept of biomarkers, spotlighting their diverse forms—from genetic markers to proteins to imaging indicators—and how they serve as biological clues that could help unravel the mysteries of neurodegenerative diseases.
This article covers:
What are biomarkers?
A biomarker—a portmanteau of "biological marker"—is a measurable indicator of the underlying biology of a disease. Biomarkers can identify the presence of a health condition, monitor disease progression, and evaluate how well the body responds to treatment. They also provide valuable insights into the underlying disease processes and enable researchers to track disease-related changes at the cellular and molecular level.
One of medicine's most widely used biomarkers is blood pressure, an indicator of how the cardiovascular system is functioning. Imaging test results from X-rays, CT scans, and magnetic resonance imaging (MRI) are also used as biomarkers. So, too, are blood tests such as hemoglobin A1c (HbA1c), which signals a person has elevated blood sugar levels and may have prediabetes or diabetes.
However, for neurodegenerative diseases, biomarkers have been hard to come by and so are rarely used in research or the clinic. Instead, people are diagnosed based on clinical symptoms alone, which often appear late in the disease process once damage to the nervous system has occurred. At this point, it is often too late to intervene in a disease and make a meaningful difference in a patient's life.
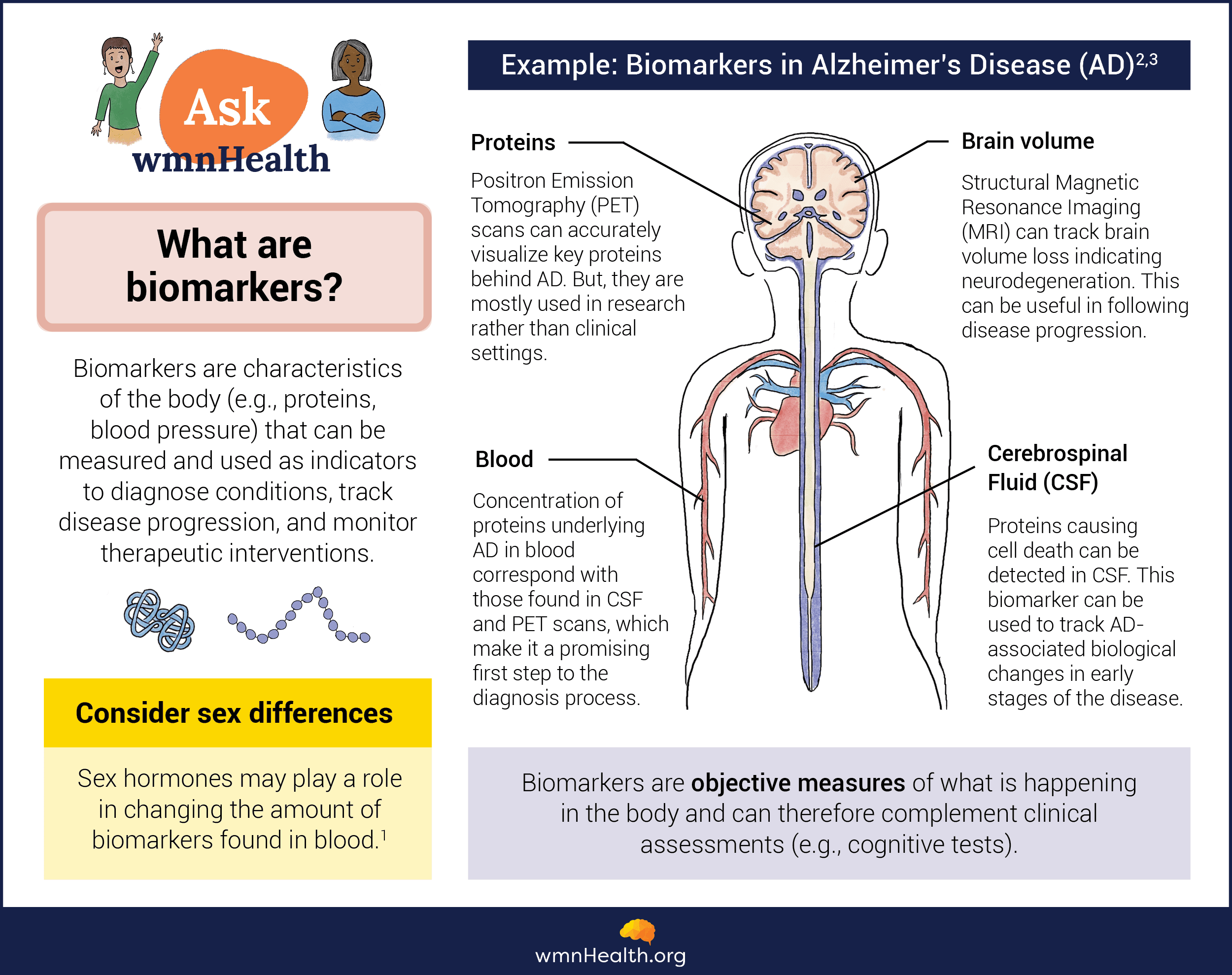 Infographic by Cat Lau. (References below.)
Infographic by Cat Lau. (References below.)Why is it challenging to find biomarkers for neurodegenerative diseases?
It is challenging to find reliable biomarkers for neurodegenerative diseases for several reasons. First, these diseases are complex, involving multiple cellular and molecular components, many of which are still undiscovered.
Second, neurodegenerative diseases are highly variable, meaning they can manifest differently among individuals. This variability complicates the discovery of universal biomarkers, which accurately reflect the disease in everyone.
Third, the brain is inaccessible relative to other organs and tissues, making it challenging to directly observe biological changes associated with brain diseases. While it is possible to get a window into the brain with imaging and cerebrospinal fluid samples, those techniques require costly, specialized equipment and expertise. For biomarkers to be useful, they must be accessible, inexpensive, and easy to apply.
Finally, brain diseases like Alzheimer's often have pathological features that overlap with normal aging processes, which makes it difficult to find biomarkers that specifically indicate disease-specific changes rather than age-related alterations to the brain.
However, despite these challenges, researchers continue to make progress on biomarker discovery for neurodegenerative diseases.
What are the different types of biomarkers?
Various types of biomarkers are used in neurodegenerative disease research, each providing unique information about the disease process. They can be broadly divided into "wet" biomarkers, which focus on indicators in the blood, serum. saliva, spinal fluid, or tissue, or "dry" biomarkers like genetic mutations, brain imaging measures, and clinical indicators.
Genetic biomarkers
Genetic biomarkers, such as specific gene mutations, can indicate an individual's susceptibility to developing a particular neurodegenerative disease. For instance, the APOE ε4 allele is strongly associated with an increased risk of developing Alzheimer's disease.
Biochemical biomarkers
Biochemical biomarkers, on the other hand, involve the analysis of specific molecules present in bodily fluids, such as blood or cerebrospinal fluid, a fluid that bathes the brain and spinal cord and is rich in glucose, proteins, lipids, and electrolytes. For example, the presence of abnormal levels of amyloid-beta and tau proteins in cerebrospinal fluid has been linked to the development of Alzheimer's disease.
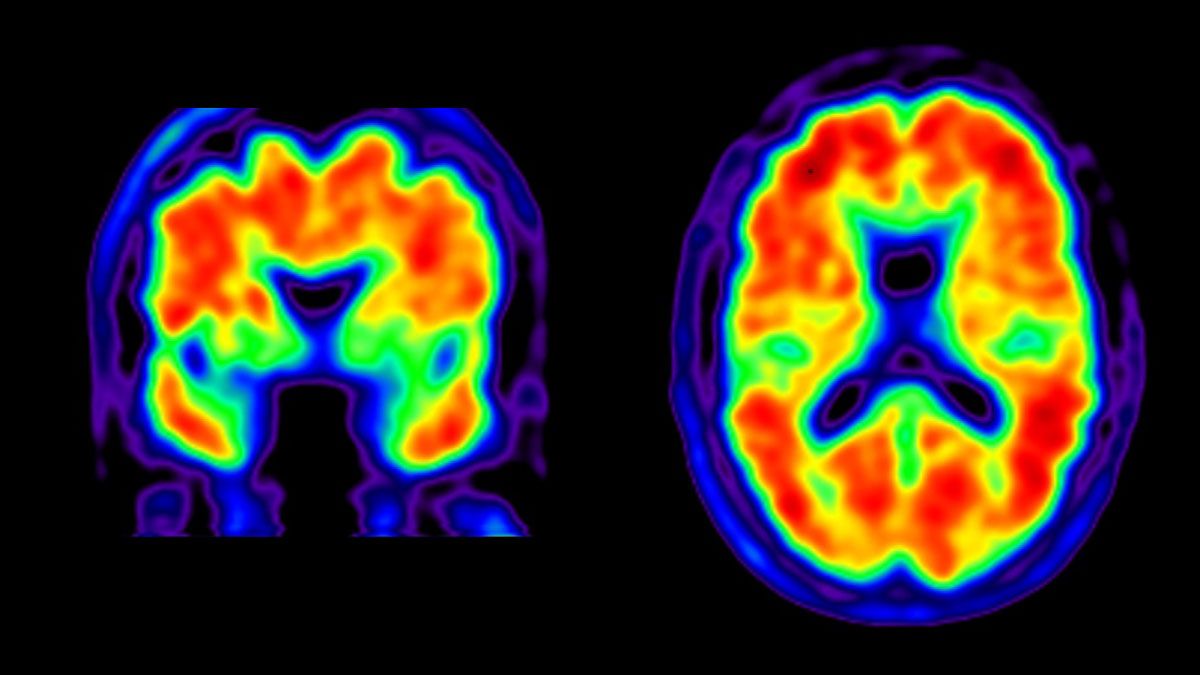 Amyloid-positive PET scans (Credit: UCSF)
Amyloid-positive PET scans (Credit: UCSF)Imaging biomarkers
Imaging biomarkers rely on advanced brain imaging techniques such as MRI or positron emission tomography (PET) to visualize molecular, structural, and functional changes in the brain. These techniques can provide valuable information about neurodegenerative disease progression, such as the presence of brain atrophy or abnormal protein deposition (pictured).
Clinical biomarkers
Clinical biomarkers, such as cognitive, motor, or behavioral tests, are critical for a comprehensive diagnosis of neurodegenerative diseases and are usually combined with other biomarkers. For example, in Parkinson's, a physician may combine a standard scale to score a person's movements and a test for abnormal alpha-synuclein to confirm a diagnosis.
Digital biomarkers
Digital biomarkers are gaining traction in healthcare. They are objective measures of someone's health derived from data collected and analyzed using digital devices like smartwatches or tablets. They encompass a wide range of metrics, including heart rate variability, activity levels, sleep patterns, body temperature, and cognitive function.
Digital biomarkers promise continuous, non-invasive monitoring of a person's health status and early detection. However, skepticism persists about their widespread adoption and efficacy, particularly in real-world settings.
What's new in biomarker discovery research for Parkinson's and Alzheimer's?
To diagnose Alzheimer's disease, physicians still rely heavily on documenting mental decline using a person's medical history, neurological exams, and cognitive, psychological, and functional assessments. But they can also order medical tests to check for the presence of two biomarkers: amyloid beta and tau. These two proteins form abnormal brain deposits strongly linked to Alzheimer's. Amyloid-beta and tau can be detected in the spinal fluid using biochemical tests or in the brain using molecular imaging technologies like PET scans. A different set of imaging tools are also commonly used to detect changes to the structure and function of the brain in Alzheimer's.
Still, researchers are working to discover additional biomarkers for Alzheimer's that could serve as a simple, inexpensive, non-invasive way to diagnose the disease. For example, biomarkers in the blood, urine, and saliva, as well as the eye could be used to detect the disease early.
The Parkinson's biomarker announced last year uses abnormal alpha-synuclein, a protein that misfolds and forms clumps in the cells of people with Parkinson's, as an indicator of the disease. Researchers developed a tool—the alpha-synuclein seed amplification assay (ɑSyn-SAA)—to detect the misfolded protein in the spinal fluid of individuals diagnosed with Parkinson's. The tool amplifies tiny amounts of misfolded alpha-synuclein in samples from people with Parkinson's disease so the protein can be readily detected in the lab. Now, for the first time, Parkinson's can be diagnosed biologically in a living person. The tools can also detect abnormal alpha-synuclein in individuals at risk for the disease but who have not yet developed symptoms or been diagnosed.
Biomarkers for Parkinson's aren't limited to alpha-synuclein. Researchers are also exploring whether a protein called neurofilament light (Nfl), a sensitive measure of neuronal damage and an enzyme called DOPA decarboxylase could be used as Parkinson's biomarkers. Loss of smell and sleep changes are also being explored. In addition, researchers are developing tests for alpha-synuclein that can detect misfolded protein clumps in the skin or blood, making sampling much easier and cost-effective.
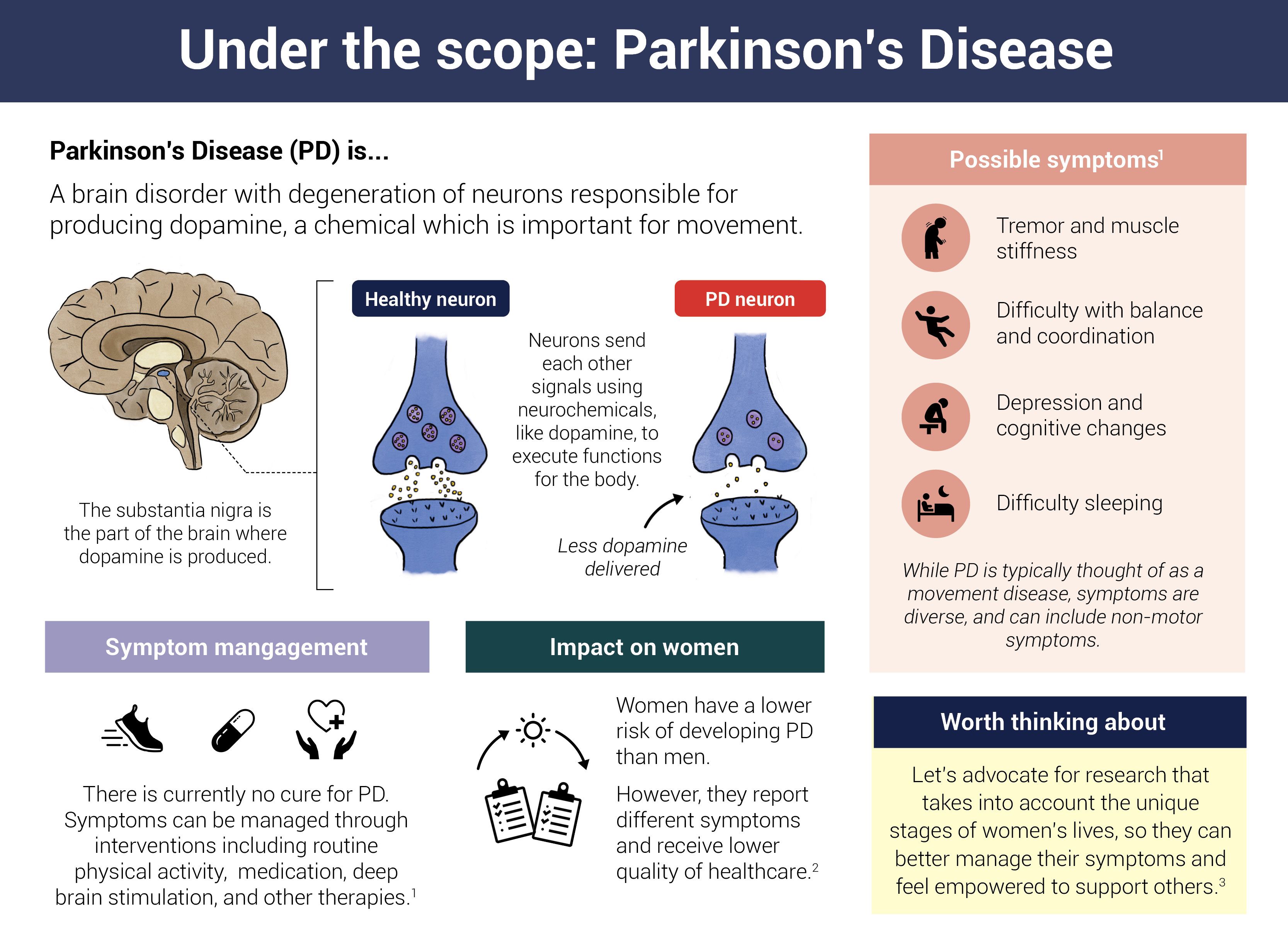 Infographic by Cat Lau. (References below.)
Infographic by Cat Lau. (References below.)How can biomarkers for neurodegenerative diseases help transform research and care?
The principal uses for biomarkers are early detection, diagnosis, and disease progression monitoring.
Early detection
By identifying biomarkers correlating with disease onset, researchers can develop screening tests that allow physicians to act before irreversible damage occurs.
A study published in The New England Journal of Medicine demonstrated the utility of biomarkers in predicting the onset of Alzheimer's disease. The researchers found that combining a genetic biomarker (APOE ε4 allele) and a biochemical biomarker (amyloid-beta protein levels) allowed them to accurately predict disease onset up to 20 years before symptoms appeared.
Furthermore, biomarkers can help differentiate between neurodegenerative diseases that may present with similar clinical symptoms. This distinction is crucial as it allows targeted treatment approaches tailored to the specific disease pathology.
Accurate diagnosis
Diagnosing neurodegenerative diseases accurately and efficiently remains a significant challenge. Biomarkers offer a promising solution by providing objective measurements that supplement clinical assessments.
For instance, the International Working Group (IWG) criteria for Alzheimer's disease diagnosis now includes biomarker evidence of amyloid-beta and tau abnormalities in addition to clinical symptoms. By integrating these biochemical biomarkers of Alzheimer's, physicians can identify it in its early stages, even before significant cognitive decline emerges.
Last month, experts published a similar system for diagnosing and staging Parkinson's disease using a combination of biomarkers, including abnormal alpha-synuclein, dopamine degeneration in the brain, and clinical symptoms like motor and non-motor disturbances. According to the proposed system, an individual with misfolded alpha-synuclein in neurons but no symptoms of Parkinson's is stage zero. As the disease develops, a person will progress through stages three to six as their functional impairment increases.
Monitoring disease progression
The ability to monitor disease progression is crucial for understanding the natural course of neurodegenerative diseases and evaluating whether potential treatments are having an impact.
By regularly monitoring biomarkers, researchers and clinicians can track disease severity changes, identify individuals more likely to benefit from specific treatments, and assess how patients respond to therapies. This type of data can also help develop and evaluate new therapies in clinical trials.
A recent study published in The Lancet Neurology demonstrated the potential of biomarkers in monitoring disease progression in Parkinson's disease. The researchers used a combination of imaging and biochemical biomarkers to track the spread of abnormal alpha-synuclein. This detailed understanding of disease progression can contribute to developing targeted therapeutics focused on halting or delaying disease progression.
Why sex-specific biomarkers are needed for neurodegenerative diseases
Neurodegenerative diseases often exhibit sex-specific differences in terms of disease prevalence, symptoms, and progression. Therefore, understanding these differences and developing sex-specific biomarkers is crucial for improving diagnosis and treatment outcomes in women and men. By identifying sex-specific biomarkers, researchers can also gain insights into the underlying mechanisms driving these differences.
For example, studies have shown that women are more prone to developing Alzheimer's disease than men, but it is not entirely clear why. There are also differences in the buildup of amyloid beta and tau in the brains of women and men. Again, researchers don't fully understand why.
One hypothesis involves the immune system. A dysfunctional immune system is emerging as a driver of Alzheimer's disease. At the same time, it is also widely recognized that women are disproportionately affected by immune disorders compared to men. Could these immune differences explain why Alzheimer's is more common in women, why the pathology looks different in women, and why women tend to decline more rapidly than men after diagnosis? Researchers are attempting to find out by identifying sex-specific biomarkers for the disease, which could include measurements of the immune system.
Biomarker discovery is leading to a fuller understanding of neurodegenerative diseases and helping to advance their diagnosis, treatment, and monitoring. However, challenges remain in unlocking the full potential of biomarkers to improve the lives of individuals affected by these diseases, including optimizing biomarkers for use in research and the clinic, practical implementation, and the need for sex-specific biomarkers.
Infographic References
What are biomarkers?
- Sex differences in biomarkers impact clinical testing, Women's Health Research Institute (accessed January 23, 2024)
- Dubois, B., von Arnim, C.A.F., Burnie, N. et al. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alz Res Therapy 15, 175 (2023).
- Klyucherev, T.O., Olszewski, P., Shalimova, A.A. et al. Advances in the development of new biomarkers for Alzheimer’s disease. Transl Neurodegener 11, 25 (2022).
Under the scope: Parkinson's disease
- Parkinson's Disease, National Institutes of Health (Accessed June 9, 2023)
- Women and PD, Parkinsons.org (Accessed June 9, 2023)
- Subramanian, I., et al. Unmet Needs of Women Living with Parkinson's Disease: Gaps and Controversies. Movement Disorders, Vol. 37, No. 3, 2022